In this image from Panama, vertical strings of flashes, each created by an individual male ostracod, linger in the water. When one male begins to display, others join in, aiming to lure females. (Photo by Trevor Rivers)
Originally posted in Science on Aug. 25, 2022 [doi: 10.1126/science.ade5292]. Reprinted with permission from AAAS.
In the 18th century, the French naturalist Godeheu de Riville was sailing across the Indian Ocean when he came upon a remarkable sight. The sea “was covered over with small stars; every wave which broke about us dispersed a most vivid light, in complexion like that of a silver tissue electrified in the dark,” he recounted in his journal. When de Riville examined the sparkling water with his microscope, he discovered that the “small stars” were tiny crustaceans now known as ostracods.
Centuries later, in 1980, marine biologist James Morin was scuba diving just after sunset in the Virgin Islands when he noticed bright blue dots blinking on and off several meters away. When he shone his flashlight through the water, he saw scores of ostracods flitting across its beam. After multiple dives, he discerned that the flashes weren’t random. The ostracods lit up in specific patterns in space and time, much like the courtship flashes of fireflies that light up summertime meadows. The realization changed the course of Morin’s career.
Now a professor emeritus at Cornell University, Morin has spent the past 4 decades working with a small, dedicated group of colleagues to unravel the mysteries of what they describe as the most spectacular natural wonder that most people will never see. Male ostracods only display for about an hour, shortly after sunset on moonless nights in warm Caribbean seas. Most recreational divers don’t dive at night, and those who do tend to use lights, which prompt the creatures to switch off for the evening.
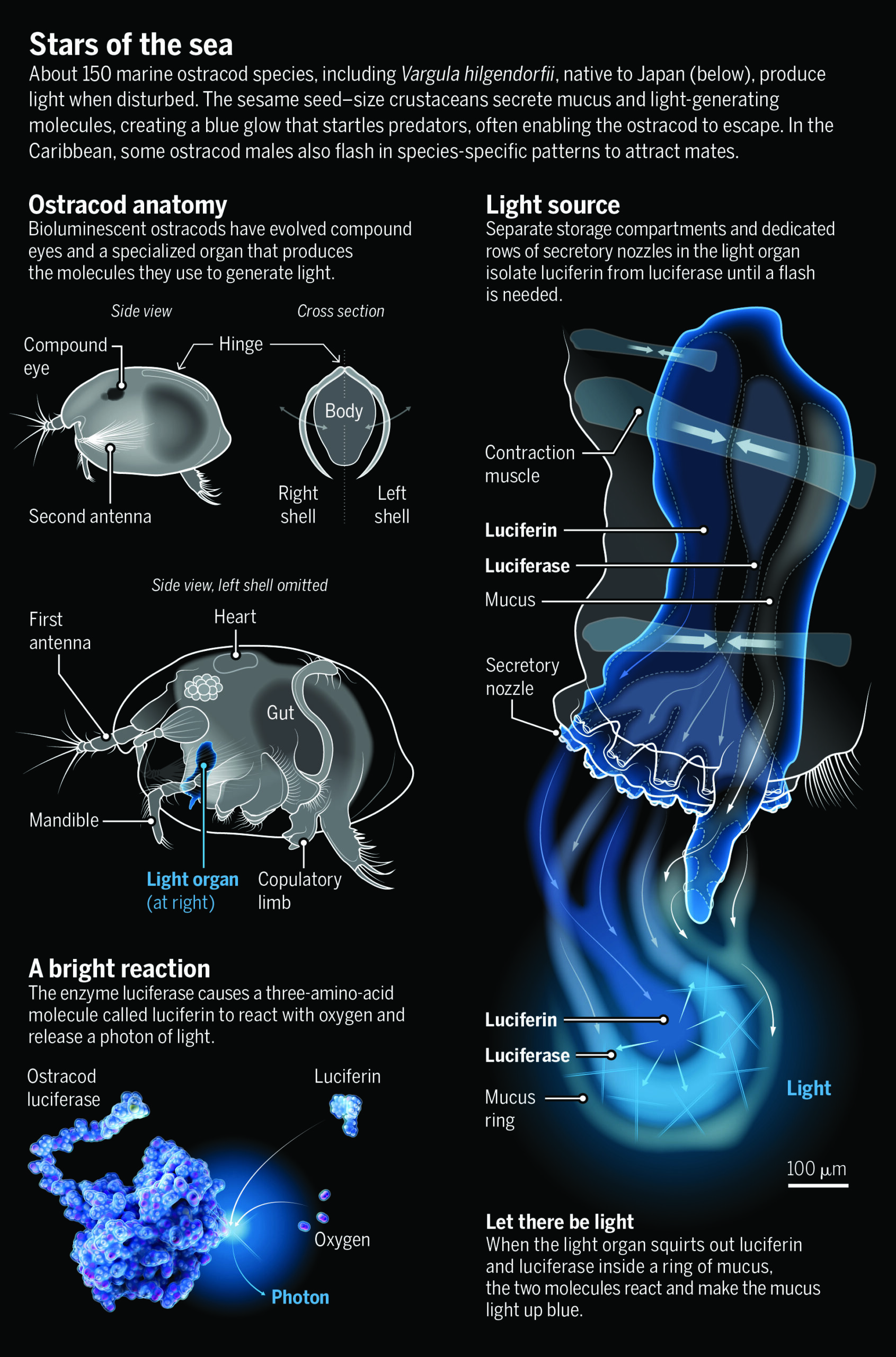
No bigger than a grain of sand, ostracods abound in fresh and saltwater. “They are very cute but also sort of bizarre—like a cross between a crab and a tiny spaceship,” says Timothy Fallon, an evolutionary biochemist at the University of California (UC), San Diego.
Only seagoing ostracods are bioluminescent, and it’s not their bodies that glow. Rather they spew out glowing mucus. In most of the world’s oceans, ostracods do this for defense—to startle and distract would-be predators. But in the Caribbean, and only in the Caribbean, as Morin and colleagues discovered, those bright blue dots can double as mating calls. Today, thousands of dives later, they believe those signals have driven Caribbean ostracods to diversify into more than 100 species.
With modern genetic tools, they’ve been using these creatures to investigate the factors that wedge species apart, including sexual selection, driven by female preferences; geographic isolation; and genetic drift—the accumulation of random genetic changes. In just the past 2 years, researchers have figured out how to grow ostracods in the lab, a development that will allow them to dissect the molecular mechanisms of evolution in a way once possible only in more conventional lab animals such as nematodes and fruit flies.
“The ability to ask interesting questions about evolutionary patterns across multiple species is a powerful tool,” says Christopher Cratsley, a behavioral ecologist at Fitchburg State University who works on fireflies. Ostracods are an “elegant system” for doing so, he says. The mechanics and biochemistry of their light flashes are relatively simple, and many species of ostracods overlap in small areas. Compared with other animals with complex mating rituals—songbirds, say—they may more readily yield clues about the forces that generate biological diversity.
IN JAPAN, dried ostracods are popular as curiosities because they glow when rehydrated. They’re called umi-hotaru—“sea fireflies”—and in the first half of the 20th century, they caught the eye of Princeton University biochemist E. Newton Harvey. He used dried ostracods to work out the basic biochemistry of bioluminescence, which has evolved independently about 100 times. Organisms as disparate as bacteria, fungi, fish, and insects use it to evade predators, attract prey, or communicate with their own kind. For several, such as fireflies, it’s a means of courtship.
In the Caribbean, the light show takes place underwater. When male ostracods sense the water is dark enough, they take off from the reef or seagrass bed where they spend most of their time and begin their display. Females swim toward the flashes, as do nonflashing males racing to intercept them.

To watch the spectacle, sea firefly researchers in scuba gear position themselves on the sea floor just after dark, using red lights to find their way. It’s an eerie experience. At night, shallow reefs resound with snapping shrimp and the crunch of parrot fish chomping on coral. Deeper waters are spookily quiet.
In the early days, biologists used a night vision monocular attached to a VHS camera to capture the displays. The images were grainy and had a limited field of view. “It was very hard to see a whole display,” says Gretchen Gerrish, one of Morin’s first sea firefly graduate students and now an evolutionary ecologist at the University of Wisconsin, Madison. The video equipment has improved, but even now, researchers often take waterproof notes by writing on pieces of PVC pipe with a mechanical pencil. A rubber band, rolled down a finger’s width after each line, helps them keep their place. After documenting a display, they swim to the flashes and scoop up the creatures with a net.
Back in the lab—often a makeshift setup in a hotel room—they sort through their catch and examine their collected ostracods under a microscope to identify the species. Sometimes a subtle difference in the shape and size of the reproductive organs or in the relative proportions of body parts is all that distinguishes one species from another. So far, they’ve named more than 20 species; about 100 more await formal description.
By the early 2000s, the work had revealed that the behavior of courting ostracods is surprisingly complex: Flashes can be dim, bright, or even different tints. They last from milliseconds to multiple seconds. And while generating them, the ostracods move in species-specific ways—up, down, or on a slant—creating strings of flashes that range in length from less than 1 meter up to 30 meters. Everywhere Morin and his colleagues went, they found new species and new behaviors. “It was really hard to comprehend the scale of unknown knowledge we were stumbling upon,” recalls Nicholai Hensley, an integrative evolutionary biologist at Cornell.
TO DELVE MORE DEEPLY into sea firefly biology, Gerrish recruited nearly all the other sea firefly researchers to study the creatures in a project that extended across five sites in the Caribbean between 2015 and 2019. They teamed up with Martin Dohrn, a filmmaker known for capturing nature in poorly lit places, to develop an underwater camera system that records visible and infrared light at the same time. That enabled the researchers to see the blue flashes as well as the animals themselves. The flashes show up as visible light, but the only way to see the ostracods without drowning out the flashes is to use infrared. “It transformed our abilities to document the displays in the field,” Morin says.
The group also began to tease apart relationships among ostracod species. Todd Oakley, an evolutionary biologist at UC Santa Barbara, and his team used RNA samples to sequence the “transcriptome,” or set of expressed genes, for Caribbean ostracod species and compare them with the transcriptomes of other ostracods, including those of the Pacific and Indian oceans, which don’t use bioluminescence for courtship. Based on the degree of similarity in the transcriptomes, they arranged each species on a family tree and used the numbers of genetic differences between species as a “molecular clock” to determine when each originated.
This work revealed that the ability to generate light, presumably for defense at first, evolved about 197 million years ago, Oakley reported in a preprint posted on 13 April. The ostracod family tree indicated evolution co-opted this defense mechanism for mating displays only once, about 151 million years ago. The early date came as a surprise to Oakley and others, who had assumed this behavior was a relatively recent innovation that arose after the Isthmus of Panama formed 3 million years ago, separating Caribbean ostracods from their Pacific kin. It’s not clear why glowing courtship displays didn’t spread to the Pacific before the barrier formed, but one possibility is that ostracods simply don’t disperse widely. Another is that visual signals don’t work as well in murky Pacific waters.
The consortium is now using ostracods as a new lens on one of the biggest questions in evolutionary biology: What drives the formation of new species? Female choosiness about mates is one candidate. Such sexual selection can drive the evolution of brighter colors or bigger horns in males, but there has been little evidence it can actually split one species into two. Oakley thinks his studies of bioluminescence provide some of the first proof. He and his student Emily Ellis compared the rates at which new species form in ostracods that use their flashes as courtship displays with the rate in those that don’t. In principle, new species should arise more frequently in populations where sexual selection is at play. And they do, Oakley’s team reported in 2016—not only in Caribbean ostracods but also in insects, fish, and octopuses with bioluminescent courtship displays.
Gerrish, too, has found evidence in ostracods that sexual selection can drive diversity. She and others had observed that when multiple ostracod species live close together, individual species’ displays become more distinctive than when the same species are found on their own, which maintains diversity by making coexisting species less likely to crossbreed.
At a study site in Belize, Gerrish and her graduate student Nick Reda have also found evidence that distinctive displays might be pushing one species to divide into two. Most males of the species Photeros annecohenae swoop upward as they flash, painting their string of glowing dots in a consistent direction. But a few swoop downward, and this tendency seems to be increasing, suggesting enough females prefer this behavior to cause it to proliferate. In one seagrass bed along the reef where Gerrish has been working, 50% of the males now exhibit this odd behavior, and in an adjacent bed, that percentage has grown to 70%, she and Reda plan to report in a paper later this year. As this mating display becomes more common, it’s more likely to isolate this population, a key step toward speciation.
On that same reef, Gerrish and Reda see hints that other well-known evolutionary forces are at play. Multiple populations of P. annecohenae that live along the reef exhibit a genetic gradient, with quite distinct populations at either end, she, Reda, and colleagues plan to report later this year. They think random genetic drift is driving some of the differences. But geographic isolation also seems to contribute. In places where storms have created short breaks in the reef, isolating populations on either side, genetic differences are greater than expected from the gradient.
INCREASINGLY, ostracod researchers are delving into how evolution shaped the animals’ most distinctive feature—their flashes. Oakley and Hensley, in collaboration with Elizabeth Torres, an evolutionary biologist at California State University, Los Angeles, have sequenced the ostracod genes for luciferase, the enzyme that adds oxygen to a molecule called luciferin to make light in all bioluminescent organisms. “Every new species we look at, it is a new gene,” Oakley says. Moreover, those tiny differences in the ostracod luciferase “correlate with different types of signals,” as this enzyme’s activity can dictate the brightness, duration, and other features of each flash. The findings show how novelty at the molecular level can lead to behavioral changes that could foster the emergence of new species.
Such studies would be easier in the lab, but ostracods “are notoriously difficult to culture,” says Trevor Rivers, a behavioral ecologist at the University of Kansas, Lawrence, who was an early student of Morins. The animals are very picky about temperature and light and dark regimens, and researchers aren’t even sure what most species eat. And their relatively slow life cycle, on the order of months, means a long wait to see whether the animals will breed in a particular setup.
To tackle this challenge, Oakley and Jessica Goodheart, then a postdoc in his lab, focused on Vargula tsujii, a California species that is a close relative of the Caribbean ostracods but does not use its glow for mating. For more than 2 years, their team tweaked the aquaria and the water flow, tried different feeding regimens (chicken livers proved better than white fish), and adjusted the number of ostracods per tank. In 2020, the researchers finally succeeded in getting a reproducing population. They and others hope to eventually do the same with Caribbean ostracods. But already, Gerrish says, “We’re poised to run in a bunch of different directions” to learn more about these creatures and the evolutionary forces that shaped them.
Using lab-grown ostracods, Oakley’s team has now uncovered evidence for a key idea about the evolution of novelty: that new traits often spring from modifications of existing ones. To control the secretion of the luminescent mucus, they found, ostracods rely on genes similar to those thought to be active in venom glands in centipedes and wasps, his graduate student Lisa Mesrop reported at a meeting this winter. Thus, when ostracod bioluminescence arose 197 million years ago, it wasn’t newly invented but rather a novel application for an existing gene network.
Another student in Oakley’s lab, Emily Lau, has found ostracods and terrestrial fireflies evolved the same mechanism for regulating light-generating luciferin, most likely because chemical constraints limit what cells can do to control this very reactive molecule. Both sets of organisms stabilize luciferin by adding sulfur to its chemical structure, even though the sulfur-adding proteins are very different, she has found. Evolution appears to be “constrained” to one solution, Lau says, suggesting the same mechanism is at work in other bioluminescent organisms as well.
With ostracods now reproducing in the lab, researchers hope to finally sequence the entire genome. The ostracod genome is longer than our own and has repetitive regions, and other complexities that make it challenging to sequence from single specimens snared at sea. Success in the lab would pave the way for genetic engineering not yet possible in most multicellular bioluminescent organisms, Hensley says. He’s interested in why ostracods have hundreds of luciferase-like genes. By modifying these genes, he hopes to learn how they might fine-tune light production and whether they have additional functions.
The genome might also enable researchers to track down the enzymes that make luciferin and modulate its release. Comparing those enzymes with their counterparts in other bioluminescent organisms could provide “an opportunity to figure out what’s truly necessary to make bioluminescence,” Hensley says. That’s just one of many insights de Riville could never have imagined centuries ago when he marveled at those shining stars in the sea.